 CHAPTER 4 NOTES
CHAPTER 4 NOTESThe Development
of a New
Atomic Model
 CHAPTER 4 NOTES
CHAPTER 4 NOTES
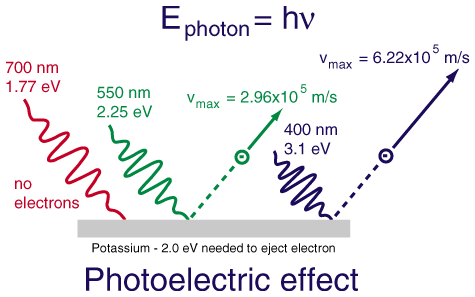
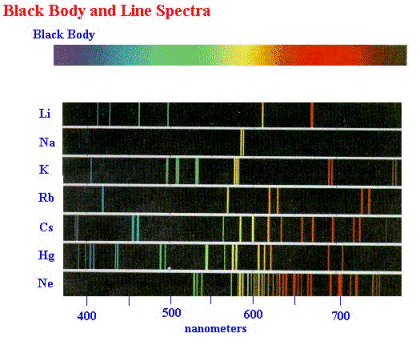
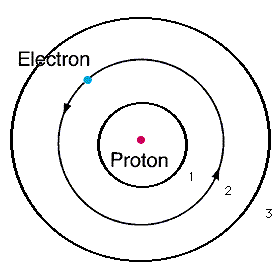
| n | l | m | s |
| 1 | 0 | 0 | + |
| 1 | 0 | 0 | - |
| 2 | 0 | 0 | + |
| 2 | 0 | 0 | - |
| 2 | 1 | -1 | + |
| 2 | 1 | 0 | + |
| 2 | 1 | +1 | + |
| 2 | 1 | -1 | - |
| 2 | 1 | 0 | - |
| 2 | 1 | +1 | - |
| 3 | 0 | 0 | + |
| 3 | 0 | 0 | - |
| 3 | 1 | -1 | + |
| 3 | 1 | 0 | + |
| 3 | 1 | +1 | + |
| 3 | 1 | -1 | - |
| 3 | 1 | 0 | - |
| 3 | 1 | +1 | - |
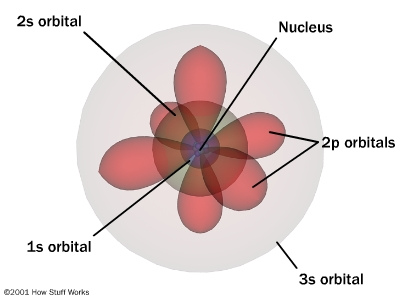
There are some natural divisions in the table. i.e. between the 1 and 2, and 2 and 3 in the first column. You could also further divide the table by separating between the 0 and 1 in the second column.
| n | l | m | s |
| 1 | 0 | 0 | + |
| 1 | 0 | 0 | - |
| 2 | 0 | 0 | + |
| 2 | 0 | 0 | - |
| 2 | 1 | -1 | + |
| 2 | 1 | 0 | + |
| 2 | 1 | +1 | + |
| 2 | 1 | -1 | - |
| 2 | 1 | 0 | - |
| 2 | 1 | +1 | - |
| 3 | 0 | 0 | + |
| 3 | 0 | 0 | - |
| 3 | 1 | -1 | + |
| 3 | 1 | 0 | + |
| 3 | 1 | +1 | + |
| 3 | 1 | -1 | - |
| 3 | 1 | 0 | - |
| 3 | 1 | +1 | - |
Notice how the chart is now broken into a group of two, another group of two, then a group of six, another group of two and then another group of six.
If you were to look at a periodic table and slide the helium over to be next to the hydrogen and then proceed to read the chart from left to right you will notice that it is also set up to be a group of two, another group of two, then a group of six, another group of two and then another group of six.
What it comes down to is that quantum numbers are actually a mathematical representation of the periodic table.
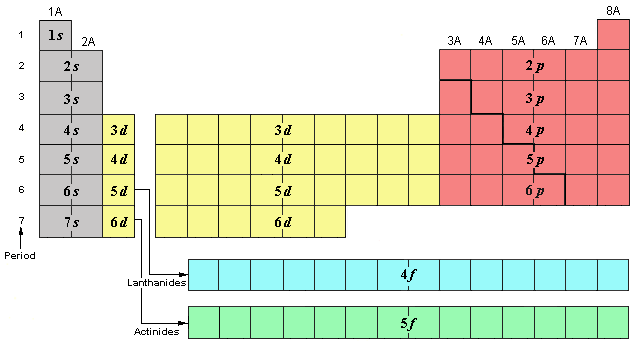 The periodic table is broken into regions according to the quantum number table that we had earlier. The n value is called the shell number, the value of l is related to the subshell where 0=s, 1=p, 2=d, and 3=f. A coefficient is then added to show the position of that element within the subshell.
The periodic table is broken into regions according to the quantum number table that we had earlier. The n value is called the shell number, the value of l is related to the subshell where 0=s, 1=p, 2=d, and 3=f. A coefficient is then added to show the position of that element within the subshell.

Just writing this information gives the location of that element, or its address. However, to give the full correct electron configuration you need to include all of the completed shells below it.
An example is oxygen which is the fourth member of the 2p subshell so its address is written: 2p4. Its overall configuarion would be 1s22s22p4.
Notice how the coefficients add up to eight and the atomic number of oxygen is eight, so you know you have it set up correctly.

Noble Gas Notation is a short cut method of the regular ECN. You go to the Noble gas that occurs before the element that you are looking for and place it in brackets. You then continue with the normal ECN until you get to the element you are looking for. An example of cobalt is shown.
This notation is particularly useful when showing high numbered elements.
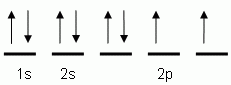
Orbgital notation is similar to ECN except that it breaks everything into its subshells and then represents every electron with its spin. A positive spin is an upward pointing arrow and a negative spin is a downward pointing arrow.
The example shown is for oxygen. Notice that there are eight arrows, and the atomic number for oxygen is eight. Also notice that the 2p orbital has two lone electrons instead of a second pair of electrons. This is becuase it must satisfy Hund's rule which states I have to have one electron in every orbital in a sublevel before a second one can be placed.
This is a method of showing how many electrons are available in a particular atom. It can be represented as follows:
| 3 | 6 | ||
| 1 | X | 4 | |
| 2 | 7 | ||
| 5 | 8 | ||
In this diagram the x represents the element symbol and the numbers are where dots would be placed to represent electrons. For example, hydrogen would look like this.
| • | H | ||
And oxygen would look like this:
| • | • | ||
| • | O | • | |
| • | |||
| • | |||