 |
Earth History |
 |

 |
Earth History |
 |

Radioactivity or the breaking apart of an atomís unstable nucleus results because the forces in the nucleus are not strong enough to hold the protons and neutrons together. There are three types of radioactivity.
An alpha particle consisting of two protons and two neutrons is emitted from the nucleus of an atom. The emission of an alpha particle reduces the mass number by four and the atomic number decreases by two.
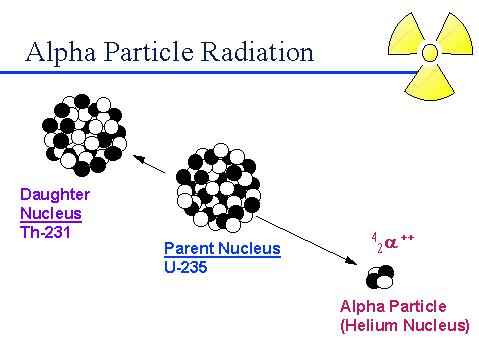
Alpha Particle Emission
A beta particle or electron is given off during beta radioactive decay. The electron given off is derived from a neutron in the nucleus which results in an extra proton increasing the atomic number of the atom by one.
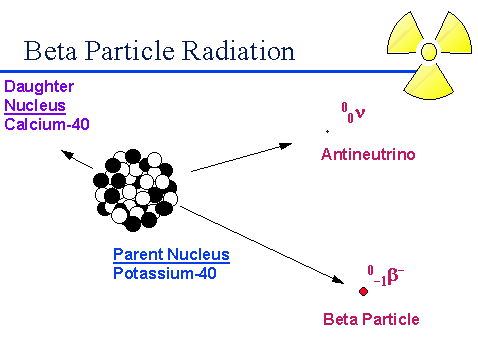
Beta Particle Emission
Electron capture radioactivity occurs when an electron is captured by the nucleus of an atom. The captured electron combines with a proton to form a neutron causing the atomic mass to decrease by one while the mass number remains the same.
Since radioactive elements decay in characteristic predictable patterns, scientists measure the percentage of radioactive parent material and the daughter products to mathematically determine the age of a rock formation. Rubidium-87, thorium-232, potassium-40, and two isotopes of uranium are used to date rocks millions of years old. Carbon-14 is used in radiocarbon dating to date recent geologic events because its half-life is only 5,730 years.
Back to Earth History Page 3 |
Earth History Page 5 |
Go To Earth History Assignments |